Investing in C3® International, Inc.
C3® International, Inc. has been formed to play a leadership role in the emerging medical cannabis health sector. C3® is a developer and provider of our Patent-pending nutraceutical. Idrasil™ is a breakthrough pharmaceutical grade pain option for the medical industry in cannabinoid therapeutics.
Company Snapshot
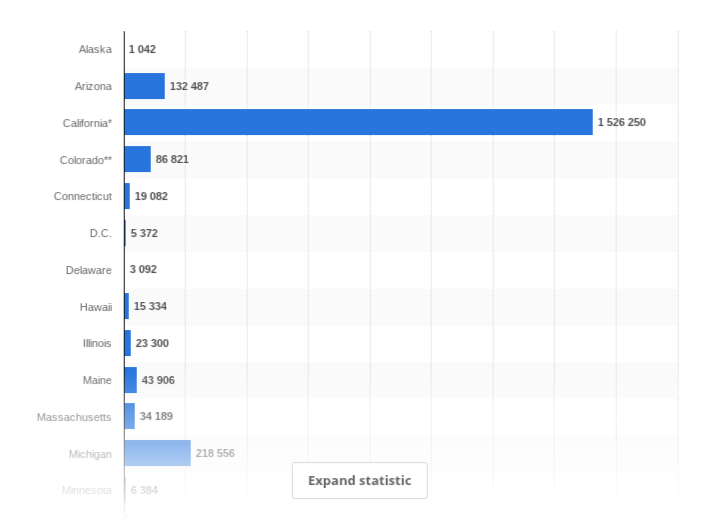
Number of medical marijuana patients in the US
This graph shows the number of legal medical marijuana patients in the United States, by state, as of August 2017. It was estimated that California had the highest number of legal medical marijuana patients with some 1.53 million.
*Source: https://www.statista.com/statistics/585154/us-legal-medical-marijuana-patients-state/
Investor Questions
Overview Snapshot of Idrasil
Idrasil™ is the first all-natural extracted cannabis pill. It contains all natural phytocannabinoids containing THC and CBD in a sterile, standardized and consistent pill. This pill is a form that physicians can feel comfortable writing on their prescription pad. That prescription can be reimbursed by most insurance companies except for Medicare and Medicaid in California. All private insurance is reimbursable.
Please go into detail about reimbursement.
All Idrasil™ sales are cash. However, historically we have used a set of codes known as ABC codes or alternative billing codes. We have successfully acquired and registered for a set of codes that cover the various strengths of Idrasil. We have submitted to our third party billing provider a claim for reimbursement and have had that claim successfully adjudicated. We are replicating that process. The only gap between us and that process now is the world knowing about Idrasil. Our stage 2 funding is wholly for advertising and sales and marketing, TV ads and our new website. We are very proud of what we feel is a world-class portal for science and cannabinoid therapeutic information.
Please give us some history of the company’s financial structure.
We’re in our Series A round of funding. Our first round has completed the end of 2017. We raised $2M plus a $0.5M contribution from myself personally. $2.5M got us to proof of concept, build out of our manufacturing facilities, our websites, our marketing, plan, and, we have a sales rep team.
What is the market opportunity and what are the initial consumer therapeutic markets you’re going to enter?
The primary markets we’re going after are pain, nausea, [and] nausea in oncology markets related to chemotherapy. Third would be insomnia, and fourth would be the psychological issues markets, depression, and anxiety patients. On our website under the doctor's information section at www.idrasilrx.com, we feel we have world-class information, both anecdotal and links to recent studies for a variety of ailments in which cannabis can be beneficial.
How is the consumer market in California growing?
In California, the [cannabis] market is currently $2B. We have a large baby boomer population that is realizing that cannabis actually has value, and if their doctor is willing to write it in a form that they can get it reimbursed by their insurance most baby boomers are comfortable with cannabis in a pill.
In the medical community, there’s obviously some significant debate over this therapeutic option.
There’s a new debate about cannabis. There is a new indication called clinical endocannabinoid deficiency, CECD. Now what that basically says is that since cannabinoids were removed from the human condition in about the 1920s, prior to the ‘20s humans used cannabis, primarily hemp in many forms, in oils, food, the seed, rope, paper, medicine. Cannabis was integral to the human condition. Now the clinical endocannabinoid deficiency theory further goes on to state that cannabinoids are the fuel to the immunodeficiency system.
Now if cannabinoids are removed from the human condition in the ‘20s and we have now 2, 3, 4 generations of humans who are clinically endocannabinoid deficient, one can now see how potentially these ailments that are effectively a ghost in the system. Like fibromyalgia, Parkinson’s, multiple sclerosis, cancer, these are diseases that the medical community is putting labels on conditions that could potentially be clinical endocannabinoid deficiencies first. Many of the ailments that I just named are at least maintained, if not helped in some way by cannabis. This is too exciting. This is too revolutionary to ignore and this is where I believe the future frontier for cannabis discussion truly resides.
What is the difference between Idrasil and Marinol, which has been available for decades?
Marinol is synthetic THC. It’s considered a delta-9-tetrahydrocannabinol molecule that replicates THC in its active form. Marinol contains no natural cannabinoids, no CBD, CBN, or CBG, or any of the other important phytocannabinoids that we recognize as having therapeutic benefits. Marinol is simply a single molecule, that after 25 years most physicians will tell you patients have reported that it does not work.
How do cannabinoid receptors connect with pain relief?
Active THC in combination with CBD works to mitigate pain in the same way opioids work on opioid receptors to mitigate pain. We have endocannabinoid receptors naturally occurring in our bodies just like we have endo-opioid receptors naturally occurring. It’s the reason why opiates work so well for pain relief, particularly musculoskeletal pain. And cannabinoids work very well with neuropathic pain. It’s just part of our physiology as humans.
Why is important for THC to be available in the pill form?
For many years, particularly in California for 20 years since cannabis has been legal [there], the medical community recognizes the anecdotal therapeutic benefit of cannabis. Too many patients for too many years have reported therapeutic benefits for a variety, a wide variety of ailments. As such, the medical community knows and is aware that cannabinoids are valuable in medicine. The problem is that cannabinoids are only available in dispensaries. Dispensaries only offer those cannabinoids typically in their raw or concentrated form, raw being the flower. These are concentrated forms that need to be smoked, and that’s the big problem with the medical community writing cannabinoid [scripts] is the lack of desire for cannabinoids to be readily ingested via the lungs. So a much safer form of ingestion already accepted by the medical community is the pill or oral ingestion, through the stomach and first pass metabolism.
As such, Idrasil is the first form of all the natural cannabinoids with active THC in a pill that’s standardized and consistent. The next point would be that consistency. When a patient goes to a dispensary and buys a bud from that dispensary, they only know the name of that bud. They don’t know where it came from, how it was manufactured, and they certainly don’t know batch to batch, lot to lot, if that medicine is consistent. Idrasil production is derived from mother genetics that is cloned, and those clones maintain the genetic integrity batch to batch, lot to lot. With our proprietary extraction and conversion process, we’re able to maintain that consistency and standardization through the physician to the patient.
A company snapshot of C3® International with Chairman Steele Clarke Smith. To learn more, visit www.idrasilrx.com or call 855-Idrasil to receive patient, doctor, and investor information.